
How does community context alter the host-pathogen interaction and the occurrence of disease?
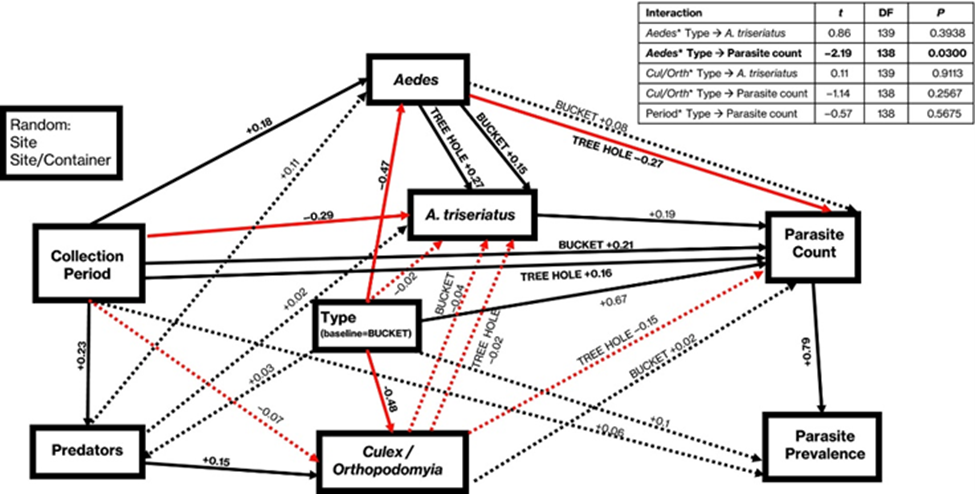
Community composition is well known to influence the occurrence of disease, with much study focusing on the impact of species gain or loss within a community. The current challenge is elucidating the relative importance of mechanisms by which changes in community composition may alter disease, to better understand how biodiversity impacts epidemics and disease emergence. My previous work approached the concept of dilution through the lens of invasion ecology (and increasing biodiversity), utilizing Aedes mosquito hosts to ask: What is the relative importance of mechanisms by which invaders may cause dilution? This work provided evidence for dilution of disease via the mechanism of encounter reduction, which was dependent on habitat type (McIntire et al. 2021). Delving further into how dilution-based encounter reduction and contact rate may impact disease in a multigenerational community, I have recently utilized Daphnia hosts to begin inquiry into theory that the dilution effect changes in magnitude and size as systems move from frequency dependent transmission to density dependent transmission.
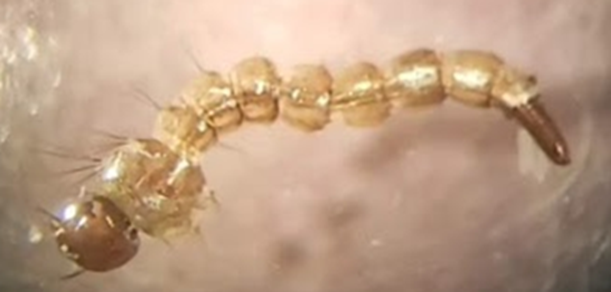
My interest in the impacts of community composition also includes how changes in the pathogen biodiversity alter disease. My work has mostly considered this through an invasion ecology perspective, which proves useful in these studies as invasion necessitates community change and yields mechanistic hypotheses which may be extrapolated to undisturbed systems. Study of parasitism during invasions is lacking, particularly the interactions of co-invasive parasites and native parasites. Understanding the interaction of co-invader and native species is especially crucial, as the impact of invasion encompass the direct and indirect effects of both the invader and the co-invader within the community. Theory predicts that, while relatively few parasites are retained during invasion, specialist parasites are more likely to become co-invaders and are more likely to spillover, yielding both disease- and competition-related impacts on native species closely related to the invader. Utilizing an Aedes host system, I was able to take steps to elucidate the mechanisms regulating these interactions and potential resulting community effects. Our results highlight the ability for these co-invaders to alter not only the native host and native parasite ecology, but also to reshape relationships within the existing native community (McIntire & Juliano 2021).
More recently, my work in this area has focused on the within host impacts of pathogen diversity on disease, outside of invasion. Though coinfections are common in nature, experimental work is most often conducted as single infections. This has left a dearth of information asking: How do pathogens interact with and within the host, altering disease emergence and outbreak? Our field data suggested that O. pajunii (a newly described pathogen; Dziuba, McIntire, et al. 2023) may shift from a low virulence to a protective, mutualistic relationship with the host, dependent on ecological context (specifically, host exposure to high virulence pathogens). Our laboratory manipulation found apparent competition between pathogens, reducing success of the highly virulent pathogen and supporting the hypothesis that pathogens may functionally become mutualists contextually. However, our results suggest that while co-exposure may yield community-level benefits to host species (in the form of reduced virulent spore production), these exposures exhibit synergistically enhanced virulence to the individual.
In the future, I will use both natural and invaded communities to investigate mechanisms by which community composition influences disease retention and transmission (building on mechanistic hypotheses identified via network analysis as in McIntire et al. 2023a). Additionally, I am particularly interested in studying with-in host “host-as-ecosystem” interactions wherein simultaneous coinfection or sequential superinfection may have vastly disparate impacts on both the host and parasite, differences that can feedback onto the larger community influencing species coexistence and community structure.
What are the transgenerational effects of parasitism and how does this impact community dynamics?
There is intense interest in understanding the degree of damage caused by a pathogen. However, despite abundant evidence that effects of stressors can carry across generations, studies of pathogen virulence have focused almost exclusively on a single generation. A focus of my postdoctoral work has been extending our conceptualization of virulence into a multigenerational framework. I did this by demonstrating transgenerational virulence, in the form of increased early mortality, in Daphnia hosts. I then collaborated with Dr. Michael Cortez (Florida State University) to construct a parameterized mathematical model, demonstrating significant population level impacts of transgenerational virulence (McIntire et al. 2023b).
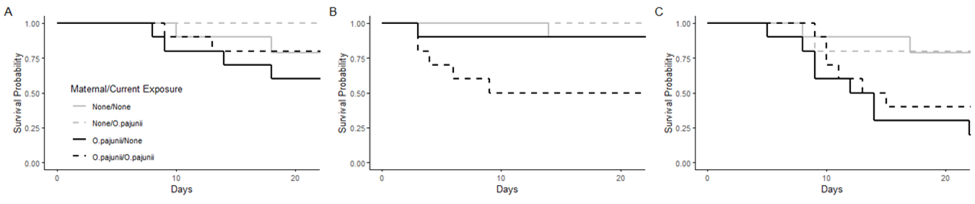
My future work will seek to build upon my findings of transgenerational effects by integrating these results into a framework that considers greater host effects to estimate relative importance of these mechanisms to the host population through both empirical experimentation and mathematical models.
In seeking to understand the causes and consequences of host-parasite interactions in nature, I utilize both a well-established disease model system and species of human medical importance. This flexibility in systems, coupled with my research questions yield work of both basic and applied significance.